Cyclopentasiloxane‘s story begins with the early days of silicone chemistry. Chemists first explored siloxane synthesis in the early twentieth century, chasing answers for materials that held up better than carbon-based options. Early siloxanes led the way for modern silicone science. Refinements in distillation, polymerization, and purification set the stage for cyclic siloxanes like cyclopentasiloxane to emerge on their own as specialty ingredients, not just side products. Major breakthroughs in the 1950s let industries push past material limitations, opening doors to new platforms in personal care, electronics, construction, and more, with cyclopentasiloxane gaining real attention as its unique volatility and silky texture stood apart from linear silicones. Over several decades, as regulatory frameworks developed and consumer goods surged, companies dialed in the processes that today bring high-purity cyclopentasiloxane to countless hands and homes.
Cyclopentasiloxane, sometimes called D5, falls into the category of cyclic siloxanes. Most folks have rubbed it into their hair or skin without knowing it, since it’s a staple in conditioners, antiperspirants, and countless skincare items. Chemically, it qualifies as a clear, odorless liquid made up of five repeating siloxane units, delivering a blend of spreadability, fast evaporation, and a light, satin feel. Unlike heavier silicones, it barely leaves residue, which sets it apart for “dry touch” finishes in modern cosmetics. Cyclopentasiloxane often works as both a carrier and a conditioning agent, walking that line between performance and comfort. Manufacturers turn to it for products needing slick, non-greasy results, especially when looking to cut down on heavy oils or mineral solvents.
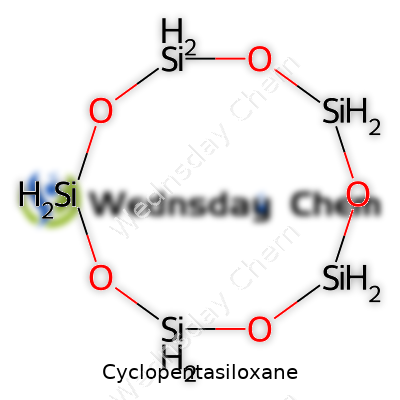
Cyclopentasiloxane’s molecular formula, C10H30O5Si5, tells part of its story. At room temperature, this compound behaves as a colorless, lightweight fluid with a density around 0.96 g/cm³ and a boiling point near 210°C. What makes it stand out is its high volatility—evaporating quickly without odor and gliding over surfaces without sticky or oily afterfeel. The substance resists water, mixing comfortably with other silicones and a range of oils, yet avoids most solvents. Chemists value its stability under neutral and mildly acidic or basic conditions, which means it survives the usual formulations of lotions and sprays. Shelf life, even in the presence of preservatives and actives, easily stretches into years.
On a technical sheet, cyclopentasiloxane usually appears with a minimum purity of 95%. Some manufacturers go even higher to satisfy the strictest requirements in pharmaceutical or electronics work. Most suppliers note key specs like refractive index (1.391), viscosity (2.2-2.7 cSt at 25°C), and flash point (77°C or higher). Product drums and bottles always carry hazard, transport, and handling marks according to GHS and local law. In Europe and North America, labels spell out the INCI name (Cyclopentasiloxane), CAS number (541-02-6), and all necessary warnings or restrictions. Transparency, traceability, and quality testing are not extras but vital routines, with barcodes and batch codes printed clearly on every carton leaving the warehouse.
To create cyclopentasiloxane, manufacturers start with dimethyl dichlorosilane, typically derived from the direct process combining silicon metal and methyl chloride. This base chemical reacts with water in controlled environments, resulting in a mixture of linear and cyclic siloxanes. The distillation phase separates cyclopentasiloxane from other cyclics like D4 or D6, using careful temperature control to sharpen purity and avoid cross-contamination. Advanced facilities harness closed-loop systems that recycle byproducts and waste, which trims costs and shrinks the environmental footprint. Continuous upgrades in catalyst and purification technology help bring cleaner, more consistent batches to market, while squeezing more value from every ton of raw silicon.
While cyclopentasiloxane stands as a stable and inert base, chemists often reach for it as a launching point for making longer silicone chains (polydimethylsiloxanes) or for blending with specialty crosslinkers and emulsifiers. Acid or base-catalyzed ring-opening polymerization lets scientists tailor viscosity and flexibility in silicone oils aimed at anything from automotive lubricants to prosthetic gels. Direct chemical tweaks—such as hydrosilylation or silanol coupling—can wrap D5 in even more functional groups, prepping it for coatings, sealants, or high-performance films. Blending with compatible volatiles or co-surfactants expands options for layered formulations, giving chemists new degrees of freedom in controlling evaporation rates or tactile effects. Each chemical sidestep rests on knowledge built up over generations of trial, error, and careful study.
In the marketplace, cyclopentasiloxane wears many hats. It shows up as D5, decamethylcyclopentasiloxane, and often simply Cyclopentasiloxane on ingredient panels. European suppliers sometimes tag it as EINECS 208-764-9 or reference its longer IUPAC name for regulatory filings. Major chemical houses sell it under various brand names—DC 345, Silsoft D5, Dow Corning 245—each with slight tweaks reflecting desired purity, viscosity, or customer segment. In Asian markets, local trade names dominate regulatory paperwork, but the molecular backbone remains the same. Clear and consistent naming remains key to safe handling and cross-industry cooperation, warding off mix-ups in transit or in large, high-volume batch work.
Anyone dealing with cyclopentasiloxane regularly spends time reviewing safety data, and for good reason. Recent years brought sharper rules, especially across the EU, Canada, and parts of Asia. Workers keep splash goggles and chemical-resistant gloves within arm’s reach, and good air flow is mandatory, particularly in enclosed production halls where its volatility can cause headaches or localized vapor build-up. Storage rules stick to dark, dry, cool rooms, away from heat or open flames given the low flash point. Fire protocols and spill kits should sit near mixing areas, since dealing with cleanup comes down to quick action and good training. Less-discussed but just as important: regulatory paperwork for cyclopentasiloxane grows every year, and routine audits mean that safety files, incident tracking, and disposal protocols must stay updated. Refusing to cut corners on compliance builds trust—both internally and with regulators and end-users.
Few synthetic molecules have traveled as far as cyclopentasiloxane. Personal care reigns supreme here—shampoos, leave-in serums, BB creams, deodorant sticks all shine brighter with D5 inside. That silky slip lets thick creams glide, mascara go on clump-free, and antiperspirants dry almost instantly. Cyclopentasiloxane also pops up in textile manufacturing, where it helps impart softness and water resistance to premium fabrics. Engineers use it in molded rubbers, electrical encapsulants, and precision lubricants, taking advantage of its thermal stability, low surface tension, and predictable evaporation profile. A few years back, I watched a group of R&D chemists in an electronics plant tune a specialized formulation for cleaning microchips, citing D5 as the lynchpin ingredient for safe, streak-free drying. Its versatility means that new application ideas keep surfacing in everything from agriculture coatings to specialty printing.
Researchers keep looking for new tricks with cyclopentasiloxane, and the last decade brought a flood of studies focused on smaller particle size emulsions, advanced drug delivery systems, and hybrid silicone-organic copolymers. Cutting-edge labs explore ways to harness cyclopentasiloxane as a smart solvent for actives that balk at water or common oils. Several universities mapped out the molecule’s behavior at the nanoscale, tracking how D5 layers interact with surface proteins, medical devices, or pigment dispersions. Collaborative work with regulatory bodies and NGOs also led to new protocols for sampling air and water residues, pushing analytical chemistry deeper and further. From my years reading industry reports, it’s clear that investments in automation, AI-assisted formulation, and green chemistry rose sharply—each new patent or academic preprint signaling a fresh take on a decades-old ingredient.
Toxicity sits squarely in the spotlight. Europe’s ECHA and Canada’s environmental authorities both published wide-ranging reviews, evaluating exposure scenarios in consumers and workers. While cyclopentasiloxane breaks down slowly in nature, studies in fish and aquatic insects found accumulations at high concentrations, which led to phase-out plans in rinse-off personal care products in some regions. Skin contact remains generally safe for most adults, with rare allergic reactions, but inhalation or chronic long-term exposure draws stricter scrutiny, especially in production or for vulnerable groups. Research teams continue building risk profiles for chronic aquatic toxicity and bioaccumulation, updating testing protocols to close knowledge gaps. Manufacturers now rethink waste management and push for technologies that catch or reuse volatile emissions before they enter the environment. Stronger engagement with toxicologists, ecologists, and public health experts brings nuance to the debates, moving toward responsible stewardship across the supply chain.
Cyclopentasiloxane faces a crossroads. Shifting regulations put pressure on brands to reduce, replace, or reformulate, especially for leave-on body care and products entering water systems. At the same time, advances in green chemistry and closed-loop production help companies stretch D5’s resource efficiency, lowering emissions far below old benchmarks. Chemists remain on the hunt for drop-in alternatives capturing the same texture magic without persistence or toxicity baggage. Startups pitch bio-based siloxanes and modified natural oils, but none quite nail D5’s balance of lightness, glide, and rapid evaporation. What seems clear is that tomorrow’s cyclopentasiloxane will travel through cleaner, safer production lines, with tighter controls and transparency from mine to makeup bag. Industry and government collaboration, paired with deeper consumer education, lights the way toward the next chapter for this remarkable molecule.
Cyclopentasiloxane pops up in all sorts of beauty products—moisturizers, serums, deodorants, hair sprays, and foundations. I remember flipping over the back of a bottle of conditioner, squinting at the small print, and there it was near the top of the ingredient list. If you’ve noticed how smoothly a lotion glides on your skin, or how hair detangles without effort, cyclopentasiloxane is often behind that sensation.
Cyclopentasiloxane is a type of silicone—colorless, odorless, and lightweight. It evaporates after application, leaving skin or hair feeling silky, never greasy. So many brands include it, not just for slip or spreadability, but also because it delivers that smooth “finished” feel we associate with premium products. Foundation settles evenly, deodorant dries quickly, sunscreen doesn’t drag during application. These qualities aren't just marketing hype; they help people use sunscreen more regularly and make grooming routines hassle-free.
Silicone ingredients have raised eyebrows. Cyclopentasiloxane is generally recognized as safe by regulatory bodies in the US, Europe, and Asia for cosmetics. That’s not a green light to slather it on with abandon. Some people with sensitive skin find that heavy use of silicone-based products can trap sweat or sebum, leading to breakouts. Personally, my oily skin has a mixed track record—some primers with cyclopentasiloxane leave my face feeling velvety, others feel suffocating on humid days.
Environmental impact deserves attention. Cyclopentasiloxane doesn’t stick around in the body, but it does linger in water. Wastewater treatment plants struggle to remove silicones, and these compounds build up in aquatic environments. In Europe, regulations now restrict its use in wash-off products, nudging companies to reformulate shampoos and cleansers. These changes came after scientific reviews showed the ingredient doesn’t break down easily after rinsing down the drain.
More people want cosmetics that respect both their skin and the environment. Manufacturers have moved toward plant-based emollients as replacements. While some replacements lack that immediately smooth finish, they pose fewer environmental issues. My own experiments with silicone-free hair products show mixed results: some still deliver shine and smoothness, but others feel heavy or greasy.
The real power lies with us as consumers. If a product promises a silky texture, check the ingredients. Those concerned with environmental impact or skin health can try alternatives and see how they perform. Some brands clearly label silicone-free options, making it easier to shop consciously.
Cyclopentasiloxane isn’t an evil chemical, and it’s solved plenty of cosmetic problems over the years. Users and companies face a new challenge—balancing performance, safety, and sustainability. Ingredient transparency, advances in green chemistry, and informed personal choice help keep the beauty industry heading in the right direction. For skin, for hair, for the planet, everyone benefits when practical solutions take center stage.
Standing in the personal care aisle, bottles line the shelves promising shiny hair, smooth skin, and easier mornings. Most of those bottles list cyclopentasiloxane among their top ingredients. It slides into shampoos, conditioners, moisturizers, and foundations, not as a futuristic compound, but to give products a feel people call "silky." Anyone who has tried a serum that never feels greasy but leaves the skin feeling soft has probably met cyclopentasiloxane.
People have concerns around what they apply to their skin, and that makes sense. Over the years, studies looked at whether cyclopentasiloxane soaks into the body, builds up over time, or causes harm. Science so far shows that this ingredient tends to evaporate quickly. It doesn’t stick around on skin or hair, which is partly why it's so popular in products promising lightweight formulas.
Groups like the Cosmetic Ingredient Review and regulators in Canada looked at its safety years ago. After reviewing the evidence, they labeled it safe in the amounts found in consumer goods. That said, the ingredient’s cousin, cyclotetrasiloxane, draws a little more scrutiny in Europe due to environmental concerns, not direct harm to people.
Everyone's skin is different. Sensitive skin, or someone recovering from a condition like eczema, may notice red or itchy spots after using formulas with silicones, including cyclopentasiloxane. While this happens rarely, personal experience always matters most. I’ve seen clients complain about outbreaks and blame the one change in their routine. The best approach is to patch-test a new product along the jaw or behind the ear before going all in.
Here's where things get trickier. Cyclopentasiloxane doesn’t just disappear from the planet after you rinse it down the drain. A 2018 review highlighted concerns that compounds from the same chemical family turn up in water systems, and some persist for a long time. European authorities flagged this as a potential environmental hazard, and some countries asked manufacturers to dial back their reliance on these silicones to cut down on possible accumulation in rivers and soil.
Many companies now look for ways to replace or limit these ingredients in “clean beauty” launches. Options like natural oils or different synthetic emollients step up as possible alternatives. Some don’t create the same slip and glide, but if enough shoppers care, brands will keep tinkering with the formulas.
Every new beauty product tells a story of balance between performance, user safety, and what’s best for the environment. As someone who spends hours talking to dermatologists, chemists, and regular folks worried about irritation or allergies, I recommend paying close attention to what your skin and hair actually need. If a leave-in helps your curls or a primer smooths your skin without issues, current research says using cyclopentasiloxane in those products causes little risk. Still, leaving room for questions means we keep pushing for safer products for people and the planet.
Smarter habits start by reading ingredient lists and staying curious—whether you stick with silky serums or try the new sulfate- and silicone-free shampoos. Listen to your own body’s reactions and remember that green chemistry only gets better if we support companies that invest in safer choices. Facts show cyclopentasiloxane earns its place for most, but the story always grows as science evolves.
Cyclopentasiloxane comes up often in the ingredient lists of shampoos, conditioners, skin creams, sunscreens, and even some deodorants. If you know much about beauty or styling products, you hear about silicones all the time—sometimes in glowing terms and sometimes in arguments that seem suspicious of their safety. The short answer here: cyclopentasiloxane is a silicone, and not just by name but by structure and performance.
Chemically, cyclopentasiloxane belongs to a family of compounds called siloxanes. These are molecules made of silicon, oxygen, and usually hydrogen or carbon atoms. Cyclopentasiloxane itself is a ring of five silicon and oxygen pairs. It's not just another synthetic ingredient; it’s one of the most common silicones used in personal care for its ability to spread easily, evaporate quickly, and leave skin or hair feeling smooth but not greasy.
Silicones in general get a lot of debate online. Some folks argue they’re bad for your skin or hair, or that they linger in the environment longer than they should. I’ve sifted through research papers and listened to chemists at skincare workshops—there’s plenty of mythmaking. It’s easy to feel wary of an ingredient you can't pronounce.
From a user’s perspective, cyclopentasiloxane actually delivers some clear benefits. In hair care, it helps detangle locks, adds a sense of lightness, and doesn’t weigh things down like some heavier silicones. On the skin, it works as a carrier, quickly delivering other ingredients, then evaporating so users don’t feel sticky. I’ve personally used leave-in conditioners and lightweight creams with cyclopentasiloxane during humid summers and never noticed buildup or irritation.
People who want eco-friendly choices worry because cyclopentasiloxane, along with other volatile silicones, can persist in water and air. Environmental scientists have shown that it can be found in trace amounts far from where it gets washed down the drain. Health agencies in Europe have even restricted its use in rinse-off products for this reason—not because it’s a direct threat to human health, but because of its persistence and accumulation in the environment.
Still, exposure through topical use seems low-risk for humans. Regulatory bodies like the U.S. FDA and Health Canada have both reviewed cyclopentasiloxane. Current studies point toward very low toxicity in typical product concentrations. Concerns about accumulation in wildlife habitats are valid, and ongoing research continues to track how much really ends up in the environment and what effects that might have.
Clear labeling and ingredient disclosure already help consumers decide what suits their values and needs. More companies now offer “silicone-free” lines for those who want to avoid certain ingredients, and researchers keep developing biodegradable alternatives that promise a lighter touch on the planet. I always recommend checking the full ingredient list and getting informed from trusted sources—especially if you have allergies or sensitive skin.
Ultimately, cyclopentasiloxane does fit the definition of a silicone. Its safety for personal use holds up with current evidence, while its environmental impact is at the center of ongoing debate. Honest conversation, backed by science, builds trust and helps everyone make choices that feel right for themselves and the world around them.
Anybody reading the ingredient list on the back of a moisturizer or foundation has likely seen cyclopentasiloxane. For years, this silicone-based liquid provided slip, spreadability, and that lightweight finish people love from their skincare and haircare. Some of the online chatter paints it in a suspicious light—will it clog pores, trigger breakouts, or leave skin red and angry? With so many products containing this compound, it’s tough to ignore these concerns—especially for those like me, who’ve had their fair share of skin frustrations.
Cyclopentasiloxane feels silky smooth, but it doesn’t soak in deeply. It forms a layer that’s breathable and tends to evaporate after a short time. The good news: large-scale studies and dermatology experts consistently report that this compound rarely causes allergic reactions or outright skin irritation. It’s not considered a comedogenic ingredient by leading organizations like the American Academy of Dermatology.
I struggled with breakouts in my twenties and ran from anything that sounded synthetic. But real clinical evidence matters more than buzzwords. A review of the Cosmetic Ingredient Review (CIR) panel findings backs up what many dermatologists have said to me and countless others: cyclopentasiloxane doesn’t clog pores in most people. In fact, it’s considered low risk for acne, especially compared to heavy natural oils that some sensitive folks actually react to.
Some people do say their skin rebels when using products with this type of silicone. Most reactions seem to be less about clogging pores or true chemical irritation and more about overlapping sensitivity, such as fragrances or other actives in the formula. In rare cases, those with very reactive skin or eczema might notice more dryness or even a burning feeling. My experience with combination skin taught me that less is often more, and patch testing makes a difference. Anytime something new caused a flare-up, turning over the label didn’t offer all the answers—context mattered.
Reading real-world stories in online skin communities, it’s pretty clear that a one-size-fits-all approach doesn’t work. People with acne-prone or sensitive skin often have a larger issue with stripping cleansers, strong fragrances, and harsh exfoliants than with cyclopentasiloxane.
Ingredients should be respected, not feared. If you suspect a sensitivity, patch test on your arm or jawline. Pick simpler formulas with fewer irritants. If you see consistent breakouts after using a certain product, take a break to see if things clear up. For persistent acne or irritation, check with a dermatologist instead of relying on internet rumors.
Cyclopentasiloxane continues to be voted safe for most users by industry experts, with few complaints in documented studies. Anyone seeking to avoid it for personal or ethical reasons will find plenty of alternatives on the shelves today. Honest conversations about science, experience, and personal skin history—plus real advice from trusted professionals—go further than ingredient scare-mongering. Taking care of skin means listening, testing, and learning what works best for your own routine.
Look at shampoos, deodorants, sunscreens, and makeup, and you might spot cyclopentasiloxane somewhere in the list of ingredients. This silicone-based compound gives products a silky, smooth feel and helps them spread easily. It evaporates quickly, which keeps skin feeling light instead of greasy. Brands use it because people love that sensory experience, not because it’s essential for health or hygiene.
Biodegradability isn’t just a buzzword anymore, it matters to lots of shoppers trying to limit what ends up hurting waterways and wildlife. Cyclopentasiloxane, known as D5 in the chemical world, often comes under fire. Its molecular structure makes it tough for bacteria and fungi to break it down. Studies show D5 is slow to degrade in soil and water, sometimes lingering for years. If a product washed down the drain into the environment, the traces could stay for generations.
One 2019 report from the European Chemicals Agency spelled out that D5 accumulates in fish and aquatic life. When these compounds build up in the food chain, they don’t just stay ‘invisible’—bigger fish and then people eat them. D5 doesn’t poison outright, but it stays in living tissue, boosting total chemical pollution. That biochemical footprint keeps growing, even if most folks have never heard the name.
I remember tossing the last of a face serum into the sink and thinking about where it goes next. Growing up near a river, I used to go fishing with my family. As pollution warnings kept popping up along the banks, we all saw the change—fewer fish, weird debris, slimy water. It hit home that everyday choices stack up downstream. Cyclopentasiloxane feels easy and harmless in the bathroom, but my own experience taught me nothing truly disappears once it circles through waterworks.
Companies claim cyclopentasiloxane doesn’t cause harm in small amounts, citing heats and air as natural breakdown agents. They say using it makes affordable, feel-good cosmetics. Meanwhile, watchdog groups counter that “trace amounts” multiply when millions of people use the same formulas. Research by the Stockholm Convention points out potential links to hormone disruption and long-term environmental buildup.
Regulators in Canada and the EU have placed limits or called for phase-outs for rinse-off products. Health Canada flagged D5 as an environmental concern over a decade ago. The European Union banned it in certain cosmetics by 2020. More governments keep reviewing whether the risks stack up too high.
Brands have started using alternative emollients from plant oils or biodegradable synthetics. These don’t hang around in waterways or pile up in animals. Shoppers have more power than they realize, too. Choosing leave-on lotions, creams, and hair products with better environmental track records is possible with a quick check of labels. Talking to companies or supporting smaller brands trying to fix supply chains puts pressure on industry giants to change for the better.
There’s no perfect answer, but consciousness spreading through word-of-mouth, grassroots campaigns, and smarter science brings hope. Cyclopentasiloxane made life easier for consumers; the fallout just proves how essential it is to pay attention not just to what feels good, but what sustains life on the other end of the drain.
| Names | |
| Preferred IUPAC name | decamethylcyclopentasiloxane |
| Other names |
decamethylcyclopentasiloxane D5 cyclomethicone pentamer decamethylpentasiloxane |
| Pronunciation | /ˌsaɪ.kləˌpɛn.tə.saɪˈlɒk.seɪn/ |
| Identifiers | |
| CAS Number | 541-02-6 |
| Beilstein Reference | 1539476 |
| ChEBI | CHEBI:135226 |
| ChEMBL | CHEMBL15874 |
| ChemSpider | 54639 |
| DrugBank | DB01835 |
| ECHA InfoCard | 07eaf6e4-149b-4575-9edb-83448214397b |
| EC Number | 208-764-9 |
| Gmelin Reference | 110502 |
| KEGG | C09454 |
| MeSH | D005438 |
| PubChem CID | 10731 |
| RTECS number | GV4560000 |
| UNII | JGL39QUT8I |
| UN number | UN1160 |
| Properties | |
| Chemical formula | C10H30O5Si5 |
| Molar mass | 370.77 g/mol |
| Appearance | Clear colorless liquid |
| Odor | Odorless |
| Density | 0.956 g/cm3 |
| Solubility in water | Insoluble |
| log P | 6.58 |
| Vapor pressure | 0.4 kPa (at 25 °C) |
| Acidity (pKa) | 13.6 |
| Basicity (pKb) | 13.6 |
| Magnetic susceptibility (χ) | -9.0e-6 cm³/mol |
| Refractive index (nD) | 1.396 |
| Viscosity | 2.5 cSt |
| Dipole moment | 1.17 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 338.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1556 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -8356.7 kJ/mol |
| Pharmacology | |
| ATC code | D05AX |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H413: May cause long lasting harmful effects to aquatic life. |
| Precautionary statements | PBT: Observe the label precautions. Avoid contact with skin and eyes. Do not breathe vapour. Use only with adequate ventilation. Keep container closed. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | 77°C |
| Autoignition temperature | ~235 °C |
| Lethal dose or concentration | LD50 (Rat, oral) > 24134 mg/kg |
| LD50 (median dose) | LD50 (median dose): >24 g/kg (oral, rat) |
| NIOSH | CYG |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0 – 10 |
| Related compounds | |
| Related compounds |
Hexamethyldisiloxane Decamethylcyclopentasiloxane Octamethylcyclotetrasiloxane Polydimethylsiloxane |